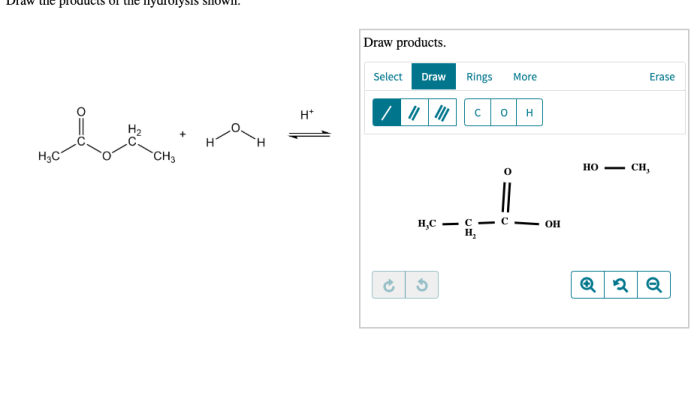
A chemist measures the energy change during the following reaction – A chemist measures the energy change during a reaction to determine the enthalpy change, which is the difference in energy between the reactants and products. This information can be used to predict the spontaneity and equilibrium of chemical reactions, as well as to design industrial processes.
Calorimetry is a technique used to measure energy changes during chemical reactions. A calorimeter is a device that measures the heat released or absorbed by a reaction. The heat released or absorbed is then used to calculate the enthalpy change.
Thermochemical Calculations
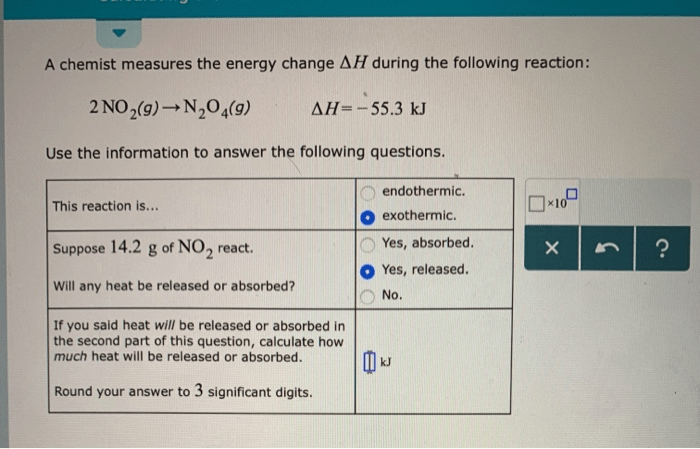
Thermochemical calculations involve the study of energy changes accompanying chemical reactions. Enthalpy change (ΔH) is a measure of the energy change that occurs during a reaction, and it can be either positive (endothermic) or negative (exothermic).
Thermochemical equations represent chemical reactions and include the enthalpy change associated with the reaction. Hess’s Law can be used to calculate the enthalpy change of a reaction by combining the enthalpy changes of individual steps in the reaction pathway.
Experimental Methods for Measuring Energy Changes
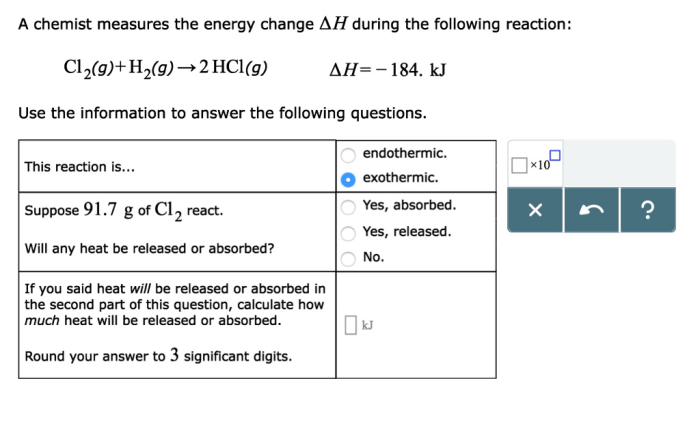
Calorimetry, A chemist measures the energy change during the following reaction
Calorimetry is a technique used to measure energy changes during chemical reactions. Calorimeters are devices that measure the heat released or absorbed by a reaction.
- Bomb calorimeters measure the heat released in combustion reactions.
- Solution calorimeters measure the heat released or absorbed when a substance dissolves in a solvent.
Calorimetry experiments involve carefully measuring the temperature change of the calorimeter and the mass of the reactants and products. The enthalpy change of the reaction can then be calculated using the specific heat capacity of the calorimeter and the mass and temperature changes.
Applications of Energy Change Measurements
Energy change measurements are used in various applications, including:
- Determining the enthalpy of formation of compounds.
- Predicting the spontaneity and equilibrium of chemical reactions.
- Optimizing industrial processes, such as combustion and fuel efficiency.
Enthalpy of formation data is crucial for predicting the feasibility and direction of chemical reactions.
Limitations and Sources of Error: A Chemist Measures The Energy Change During The Following Reaction
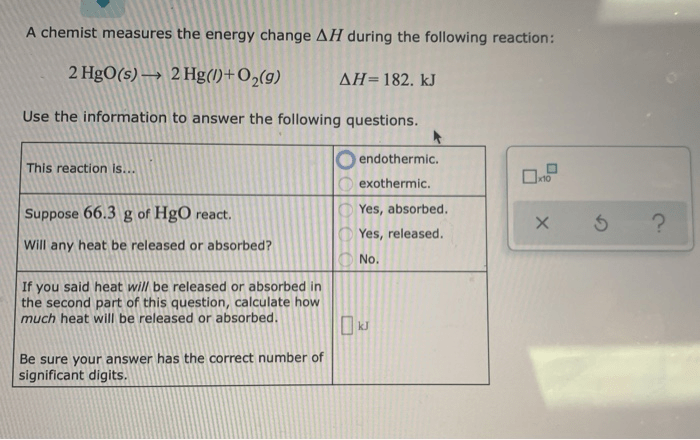
Calorimetry experiments can be subject to various sources of error, including:
- Heat loss to the surroundings
- Inaccurate temperature measurements
- Incomplete reactions
To minimize errors, it is important to carefully calibrate the calorimeter, use appropriate insulation, and conduct multiple trials.
In some cases, alternative methods for measuring energy changes may be necessary, such as electrochemical cells or spectroscopic techniques.
FAQ Summary
What is enthalpy change?
Enthalpy change is the difference in energy between the reactants and products of a chemical reaction.
How is enthalpy change measured?
Enthalpy change can be measured using calorimetry.
What are the applications of enthalpy change measurements?
Enthalpy change measurements can be used to predict the spontaneity and equilibrium of chemical reactions, as well as to design industrial processes.